|
Several recent studies have begun to explore the neurobiological basis of
serial learning and memory in rats. They suggest how serial learning
processes might be integrated into more general neurobiologically based
models of learning and memory. In one study, Olton, Shapiro, and Hulse
(1984) tested rats' sequential
memory. Four quantities of food 14, 7, 1, and 0 pellets of food were
placed in the goal boxes at the ends of the four arms of a plus maze.
Rats were allowed to choose freely among the arms and over a period of
days learned to choose the large quantities of food first and the smallest
quantities of food last. Thus rats had encoded a stimulus alphabet of
four elements and had also learned an orderly response to the four
elements as they were distributed in four spatial locations represented by
the four goal boxes of the plus maze. Once rats had learned this task,
they were given lesions of the fimbria‑fornix (FFx), the major extrinsic
pathway of the hippocampus. Subsequent testing showed that the rats could
remember to search out the quantities in the order in which they had
previously learned them. In other words, rats would go first to 14, then
to 7, then to 1, then to the arm containing 0 pellets of food. However,
if before given a free choice in the maze the rats were required to sample
one or more of the quantities out of order in a forced choice procedure,
they subsequently failed to remember having sampled the quantities when
they were tested in the free choice test. For example, if a rat were
allowed to retrieve the 1‑pellet quantity before being given the free
choice test, when the rat was allowed to make a free choice among the
arms, the rat went first to 14, then to 7, then to 1 just as if it had
never sampled the 1‑pellet quantity in the preexposure. These kinds of
mistakes indicated that rats had no memory for previously sampling food
quantities from the maze before the free choice. However, subsequent
tests showed that rats could remember elements sampled in preexposure as
long as the quantities were received in the order in which they had
originally learned them. For example, if a rat was first preexposed to
the arms containing 14 and 7 pellets of food, when given a free choice,
the rat would not run down the arms previously containing 14 and 7, but
would go immediately to 1 and then 0. Thus FFx‑lesioned rats could
remember elements presented in order, but could not remember elements
presented out of the order originally learned. These results are
consistent with Olton et al.’s (1984) interpretation that the impairment
produced by FFx lesions was an impairment of working memory, but not
reference memory. They are also consistent with Eichenbaum et al.’s
(1992) idea that hippocampus
mediates representational flexibility, the ability to use
declarative memories flexibly in new configurations, situations, or
tasks. This idea predicts that FFx-lesioned rats should be inflexible in
their use of sequential information learned before surgery, and therefore
they should be impaired in their ability to respond to probe situations
where patterns differed from the training pattern. Yet another
interpretation of Olton et al.’s (1984) results is consistent with the
view that hippocampus mediates item associative processes in SPL, but not
rule-induction and memory for pattern structure
(Fountain, Schenk, & Annau, 1985). According to this latter RL
view, FFx lesions spare information about pattern structure that mediates
responding according to the rule learned in training prior to surgery.
A second experiment shows that hippocampal lesions produce results
predicted by the RL view of serial-pattern learning if one views
rule-induction as a process potentially dissociable from item association
formation. Fountain, Schenk, and Annau (1985) trained rats with long
monotonic and nonmonotonic patterns created from quantities of
brain‑stimulation reward. The monotonic pattern was 18‑10‑6‑3‑1‑0 and the
nonmonotonic pattern was 18‑1‑3‑6‑10‑0. Prior to training, one group of
rats was exposed to trimethyltin (TMT), a neurotoxic organometal which
produces damage in the limbic system, primarily in the hippocampus. TMT‑exposed
rats learned the formally simple monotonic 18‑10‑6‑3‑1‑0 pattern as fast
as control rats, but learned the nonmonotonic 18‑1‑3‑6‑10‑0 pattern slower
than controls.
According to Olton et al.(1984), hippocampal damage should
have impaired working memory, but for both groups of rats reference memory
should have been intact. However, the results indicate differential
impairment for two different kinds of patterns despite the fact that
reference memory should have been intact and available for learning both
kinds of patterns. Similarly, according to Eichenbaum et al. (1992),
flexible declarative memory should have been impaired, but inflexible
nondeclarative memory should have been spared. This view suggests that
since learning for both groups involved learning to respond to a
consistent repeating pattern, learning for both should have been spared
following TMT damage to the hippocampus. The results fit best with the
notion that rule-induction processes were spared following TMT damage,
whereas item associative processes were impaired by TMT damage. These,
along with other data, suggested that item association formation is a
hippocampal-dependent process, whereas rule induction is not. A
dissociation of this sort may be difficult to model with SPAM, though
Metcalfe
(1993) has succeeded in simulating
characteristics of Korsakoff's amnesia using a closely related model,
CHARM.
In a third series of
experiments (Fountain & Rowan, 2000),
we sought additional evidence for this distinction between item
associative and rule induction processes. In the first study of the
series, rats were trained on two patterns, one which was structurally
“perfect” and a second virtually identical to the first, but containing a
single element that violated the otherwise simple structure. The Perfect
(P) and Violation (V) patterns were:
P Pattern: 123 234
345 456 567 678 781 812
V Pattern: 123 234
345 456 567 678 781 818
As before, the
digits indicate the reinforced lever for successive trials. The last “8”
item of the V pattern (underlined) was the violation element. Rats from
one group for each pattern condition were injected with MK-801 daily
before training. MK-801 is a systemically administered NMDA receptor
antagonist that blocks neuronal plasticity, known as long-term
potentiation,
|
 |
|
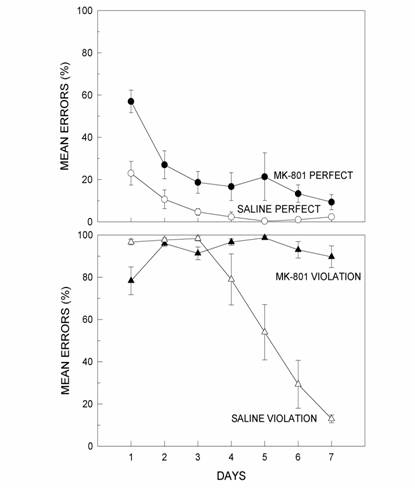
Figure 7.
Acquisition of the last element of the perfect pattern (top panel)
and violation pattern (bottom panel) over the 7 days of training
for the Saline and MK-801 groups. The last element was structurally
consistent in the perfect pattern and it was the violation element
in the violation pattern. Daily mean errors are shown for the last
element of the pattern only
(Fountain & Rowan, 2000).
|
in the hippocampus.
It is thought that MK-801 should impair any hippocampal-dependent
learning. As shown in Figure 7, MK-801 had little effect on
learning to respond to rule-based items within chunks. However, it did
impair responding at points where rules were violated, namely, on the
first trial of each new chunk and, most dramatically, for the violation
element. Although rats showed no signs of learning to respond to the
violation element, throughout the experiment they produced rule-based
errors on the violation trial by responding “2” instead of “8” at the end
of the sequence (Fountain & Rowan, 2000).
The results are strong evidence that hippocampal damage impaired learning
the item associations necessary to track violations of pattern structure
while sparing the rule induction processes necessary to induce pattern
structure and extrapolate the sequence on the violation trial.
In a later study in
the same series (Fountain & Rowan, 2000),
we examined the role of hippocampus when new serial pattern information is
added to old. Rats were first trained to a high criterion on a pattern
consisting of the first 7 chunks of the P pattern above: 123 234 345 456
567 678 781. After rats learned the pattern, they were transferred to one
of two new patterns that contained all elements of the first pattern and
an additional chunk of three additional elements. The three added
elements were either structurally consistent with the first pattern (viz.,
812), making it structurally “perfect” (P), or they contained a violation
(V) of the pattern structure learned in training (viz., 818). On the day
of transfer, half the rats were injected with MK-801 to determine the
effects of hippocampal dysfunction on the rats’ ability to integrate
structurally consistent or inconsistent new information with an already
learned pattern.
As shown in
Figure 8, when a structurally consistent chunk was added in the P
transfer, the effects of MK-801 were very similar to the effects of the
drug on acquisition (Fountain & Rowan, 2000).
That is, the drug produced a selective decrease in the animals' accuracy
on the first elements of each chunk of the original pattern, but produced
virtually no change in accuracy on the remaining two elements of the
3-element chunks. The most interesting result occurred when a
structurally inconsistent chunk was added in the V transfer. As shown in
Figure 8, although saline controls showed difficulty in learning
the new chunk, there was little effect on the rest of the pattern.
However, MK-801 dramatically disrupted performance for elements both in
the new chunk to be learned and throughout the rest of the pattern
(Fountain & Rowan, 2000).
When this effect is compared to the effects of MK-801 in the P transfer,
the effect can only be accounted for by the addition of the terminal
violation element. One interpretation of these results is that adding new
information to a pattern representation is possible under MK-801, but only
if the information is consistent with pattern structure that has already
been encoded. In fact, this initial evidence indicates that for rats with
hippocampal impairment, new information that is structurally inconsistent
can disrupt previously well-learned response patterns. This suggests
that, in intact animals, nonhippocampal systems mediate rule induction
whereas hippocampus may play a role in the successful integration of new
rule-inconsistent SPL information with already encoded information
about pattern structure. These ideas are
reminiscent of the distinction
between flexible and inflexible memory processes proposed by Eichenbaum et
al.(1992),
but our MK-801 results suggest that what constitutes “representational
flexibility” is far from resolved. Under MK-801, rats were able to add a
rule-consistent chunk to their already learned pattern with relatively
little difficulty, but not a rule-inconsistent chunk.
|