¡¡[Basics of liquid crystals]
The molecules composing solid
generally possess both positional and orientational order, while
those composing liquid do not, they can move freely in a random
fashion. In addition to the solid and liquid phase, there are some
condensed phases which show intermediate order, for example liquid
crystal.
The simplest LC is the nematic
phase, (the word nematic comes from the Greek νημα meaning thread),
the molecules are rod-like with one axis much longer than the other
two. This long axis tends to point along a certain direction, also
called director, shown in figure 1.
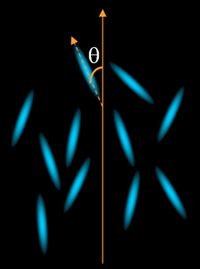
Figure 1. Nematic liquid crystal
with a preferred orientation.
Types of LC
The most common type is the
calamitic LC, with rod-like molecules, as shown in fig. 1. There
are other liquid crystal phases, which possess both positional order
and orientational order. Smectic phase is a typical one, fig ??
shows the Smectic A and Smectic C phases, where a layered structure
exists. Others like smectic B, E, F, G, H, I, J and K, have
short-range positional order or bond orientational order within the
plane of the layer; the letters denoting the chronological order of
discovery. If the molecules are chiral or doped with chiral
molecules, then a chiral nematic (smectic) phase is formed, figure 2
shows a cholesteric phase and a chiral smectic C phase.
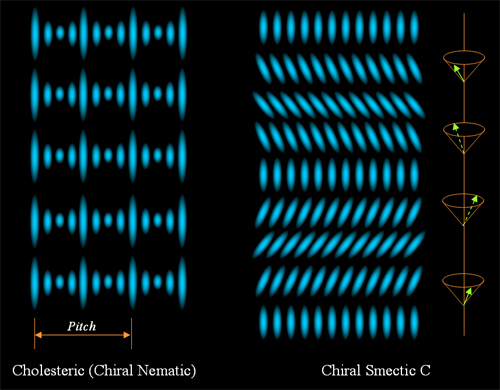
Figure 2.
Cholesteric and chiral smectic C liquid crystals
In addition to rod-like
molecules, disc-like molecules also formed liquid crystals because
of one axis is much shorter than the other two, they are called
discotic LC. Figure 3 shows a discotic nematic phase and columnar
phase.

Figure 3.
Discotic liquid crystal phases.
All the LC mentioned above are
temperature dependent, and therefore called thermtropic LC. In
contrast, LC phase can also be formed when some molecules are
dissolved into solutions, these LC's properties highly depends on
the concentration of the components in the solutions, therefore they
are called lyotropic LC. A typical lyotropic LC molecule has two
parts, one is polar and therefore hydrophilic ("like water"),
and the other is nonpolar and therefore hydrophobic ("hate water").
Figure 3 shows two typical structures of lyotropic LC.
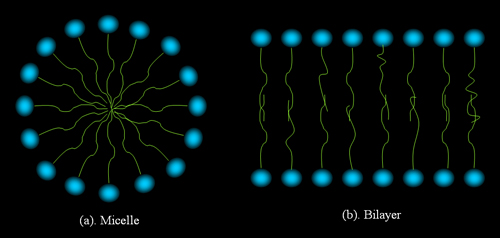
Figure 4.
Structures formed by amphiphilic molecules in polar solvents.
Electro-magnetic properties of
LC
The electric and magnetic
susceptibilities (the susceptibility of a material or substance
describes its response to an applied field) are different along the
director and perpendicular to the director. The susceptibilities
difference (anisotropy) determining the molecules' reorientation's
final direction parallel or perpendicular to the applied field: if
the susceptibilities along the director is larger than the one
normal to the director, then the molecules will align along the
applied field, vice versa.
Optical properties of LC
LC
is a birefringent medium. Light polarized parallel to the director
¡°sees¡± one refractive index, which is called extraordinary
refractive index. Light polarized perpendicular to the director
¡°sees¡± a different refractive index, called ordinary refractive
index. Therefore, if light is linearly polarized parallel or
perpendicular to the director, will experience no change to its
polarization state. However if the incoming light's polarization is
at an angle other than 0 or 90 to the director, then a phase
retardation will exist at the exit, and the light becomes
elliptically polarized. These optical properties are used in the LC
displays.
Chiral nematic
and smectic C phases are optically active, i.e. a linearly polarized light is
rotated as it passes through such a medium.
Overall, the
electro-optic properties described above (response to an applied electric field
phase retardation that causes light transmission between crossed polarizers) are
the basis for the LC displays.
Further Readings
and References:
P. J. Collings and
J. S. Patel, "Handbook of Liquid Crystal Research", Oxford
University Press (1997).
Y. S.
Chandrasekhar, "Liquid Crystals", Cambridge University Press
(1992).
P. G. de Gennes and
J. Prost, "The
Physics of Liquid Crystals", Oxford University Press (1995).
¡¡